The template is not really suitable for these purposes but some parts of the template may be used to compile an agreement for these areas. It defines the appropriate items that should be addressed in a quality agreement.
Qualitygmp standard do not need to be reiterated in the agreement.
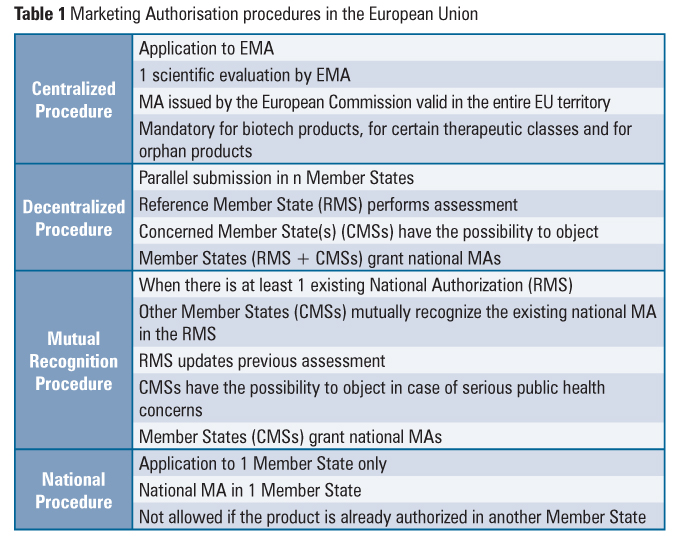
Gmp agreement template. Need for a technical agreement. It does not cover agreements between distributors and their customers and the purchase of chemicalnon gmp raw materials by the apiintermediate manufacturer. Agreement in a current state and to ensure that this agreement continues to cover the responsibilities and obligations of the parties as stated under canadian gmp regulations.
A quality agreement also known as a technical agreement and in this context the terms are interchangeable is a written contract that is required whenever a company is outsourcing an activity covered by the good manufacturing practice guidelines. By defining a clear technical agreement both parties know who will be responsible for what thus avoiding conflicts and have defined a clear escalation route. The apic quality agreement guideline and corresponding templates are designed to be a flexible model for preparing quality agreements wherever such an agreement is desired.
The ipec quality agreement guide and templates 2017. The gmp agreement is current and is not outside the review period typically applied to gmp documentation the parties to the agreement are clearly listed and have signed the agreement there is a clear and unambiguous breakdown of which entity is responsible for critical aspects of the agreement. Gmp quality technical agreements.
View standardized quality agreement templates from ipec and socma. It sets out the gmp responsibilities of each of the parties. Apic quality agreement guideline template.
A gmp technical agreement ensures compliance to the current good manufacturing practices cgmp. This document provides guidance for the implementation and maintenance of appropriate quality agreements. The format of the templates is intended to be flexible with the templates offering all the single elements needed for the compliance section of most quality agreements.
Customers whether users or distributors. This document provides guidance on how to prepare a quality agreement for pharmaceutical excipients and contains a template for it. Any modifications to this agreement must be communicated and agreed to in writing prior to implementation.










0 Response to "Gmp Agreement Template"
Post a Comment